Edaravone
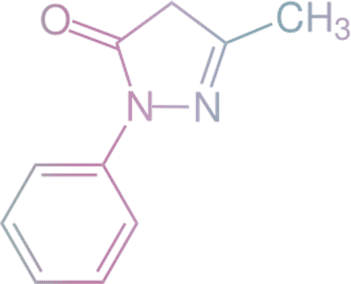

According to the international corporation “Yuria-Pharm”, edaravone is ALREADY available in Ukraine to patients with acute ischemic stroke as XAVRON.

is an ischemic cascade blocker for early empirical treatment of acute ischemic stroke.
XAVRON is a Ukrainian generic drug product identical to Radicut (Radicut®, Mitsubishi Tanabe Pharma) – the original Japanese edaravone. In the USA, edaravone was approved by FDA in 2017 for the treatment of amyotrophic lateral sclerosis as Radicava (Radicava®, Mitsubishi Tanabe Pharma).