New opportunities of using an ischemic cascade blocker in the therapy of acute myocardial infarction
Abstract. Oxidative stress plays a special role in acute coronary syndrome (ACS), in particular, is a leading link in the pathogenesis of reperfusion myocardial damage. The products of free radical oxidation can trigger the death of cardiomyocytes and are responsible for 50 % of the final size of the necrosis area in ACS, the occurrence of […]
Posted: Emergency Medicine, Volume 18, № 1, 2022
Effects of Edaravone on Muscle Atrophy and Locomotor Function in Patients with Ischemic Stroke (a Randomized Controlled Pilot Study)
Background and Objective: Stroke patients with severe leg paralysis are often bedridden in the acute and subacute phase, which increases the risk of disuse muscle atrophy in the chronic phase. The evidence to date indicates that oxidative stress plays an important role in the mechanism of disuse muscle atrophy. Therefore, the aim of this study was […]
Posted: Drugs R D 2010; 10 (3)
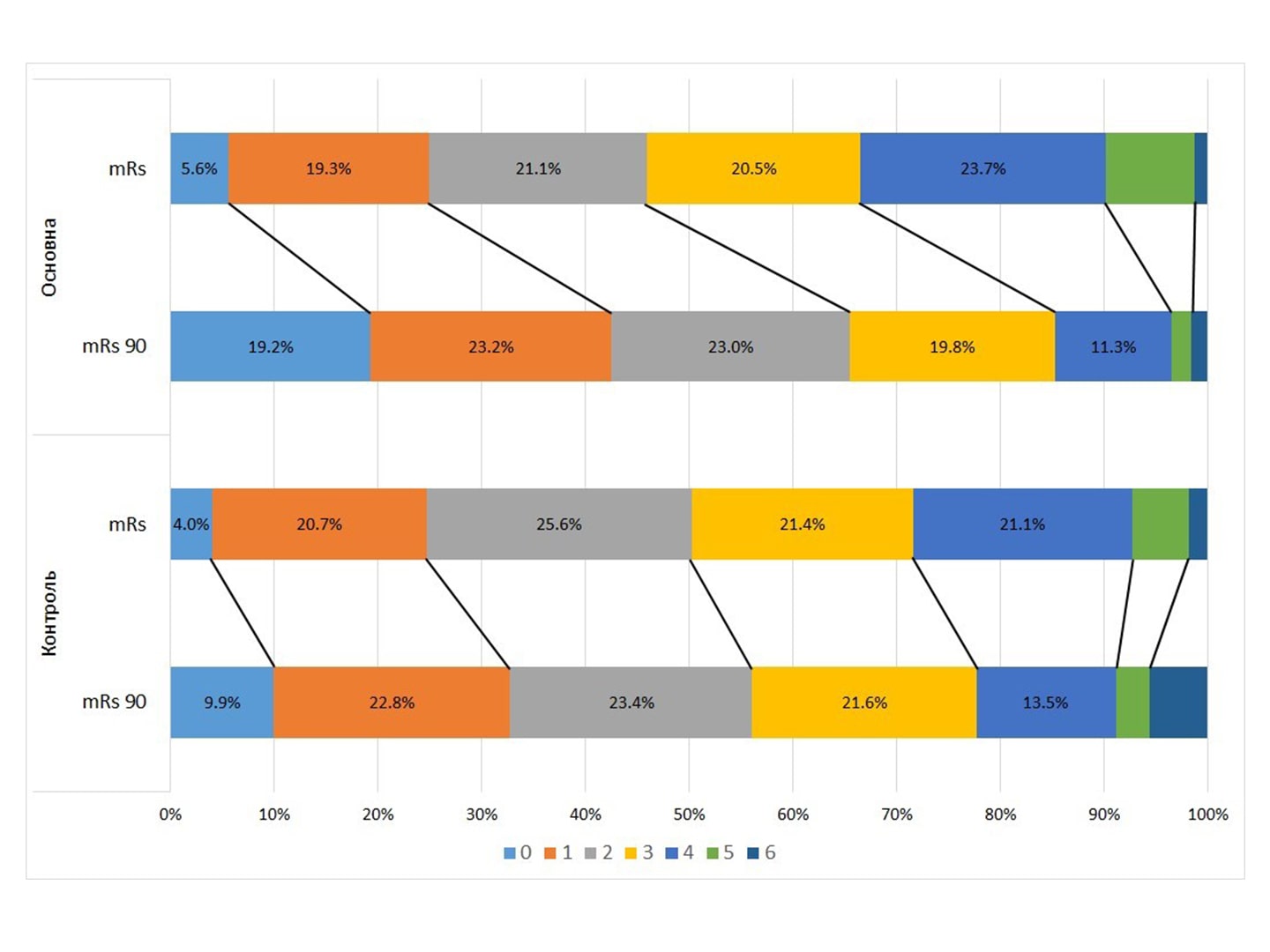
Final Evaluation of the Results of the СТІКс Study (Concomitant Xavron Stroke Therapy)
Abstract. Ischemic stroke remains a pressing problem today. Its pathogenesis consists of a sequential cascade of reactions in the brain, which, in addition to ischemia, are responsible for further damage to brain tissue and slow down the development of compensatory and regenerative mechanisms. Attempts to break the pathological cascade have been going on for decades. The […]
Posted: International Neurological Journal, Volume 17, N 5, 2021
Neurological disorders in patients who had COVID-19: how to treat during a pandemic
The article provides an overview of the world literature data on lesions of the nervous system in patients who had COVID-19. The results of an open comparative study on the effectiveness of combined use of Xavron, Tivor-L and Xylat in patients with neurological disorders in the post-COVID period are also presented. The positive effect of […]
Posted: International Neurological Journal, Volume 17, N 6, 2021
Methods of Correction of Neurological Disorders in Patients with Coronavirus Disease (COVID-19)
Eighty-three patients with coronavirus disease were examined at the Kharkiv Oblast Clinical Infectious Hospital and divided into groups depending on the therapy prescribed. In the main group, a syndromic-pathogenetic approach “Volnorez” was added to the baseline therapy. Against the background of treatment according to the scheme, which included edaravone, a combination of L-arginine and L-carnitine […]
Posted: Clinical Infectiology and Parasitology, 2021, vol. 10, no. 2
Vascular neurological complications in patients with COVID-19
The article considers modern views on the development and course of coronavirus infection COVID-19. Particular attention is paid to vascular neurological complications. Edaravone and citicoline with electrolytes were used to optimize the treatment of cerebrovascular complications in patients with concomitant COVID-19. Clinical and paraclinical data of 34 patients were analyzed. As a result, it is […]
Posted: www.umj.com.ua
The importance of non-motor symptoms in the clinical picture of motor neuron disease
Non-motor symptoms are an obligate sign of motor neuron disease and can often serve as a rather sensitive indicator of the general functional state of ALS patients. Gastrointestinal disorders, weight loss, pain, sweating and anxiety prevail in the structure of non-motor symptoms in ALS patients The presence of non-motor symptoms in patients indicates the multisystemic […]
Posted: Ukrainian Neurological Journal 2020, №3
At the forefront of the fight against COVID-19 – the experience of Ukrainian specialists (Video)
Doctors all over the world are still looking for and cannot find effective means to combat COVID-19. Therefore, every practical experience is invaluable. Let’s find out how Ukrainian doctors save the lives of the population. The latest experience was shared with leading domestic specialists in the framework of the reports of the IV International Congress […]
Posted: IV International Congress on Infusion Therapy (October 12-13, 2020)
Edaravone: A Free Radical Scavenger with Multiple Pleotropic Actions can be a Potential Game Changer Agent in Prevention and Alleviation of COVID-19 – Induced Cytokine Storm
Normal physiological process, such as cellular respiration generate small amount of oxidizing reactive oxygen species (ROS) and reactive nitrogen species (RNS). These free radicals play crucial role in activation of signalling pathways in animal and plant cells. These are chemically highly reactive molecules that can damage cell structures. However, there are endogenous defences (scavenger) or […]
Posted: Journal of Medical Science and Clinical Research Vol 08, Issue 07, Page 227-236, July
Edaravone: A potential treatment for the COVID-19-induced inflammatory syndrome?
We read with great interest the recent article by Zhong et al. titled “Efficacy and Safety of Current Therapeutic Options for COVID-19 – Lessons to Be Learnt from SARS and MERS Epidemic: A Systematic Review and Meta-analysis [1],” which is the most complete review of randomized control trials and cohort studies of potential pharmacotherapies for […]
Posted: Pharmacological Research 160 (2020) 105055
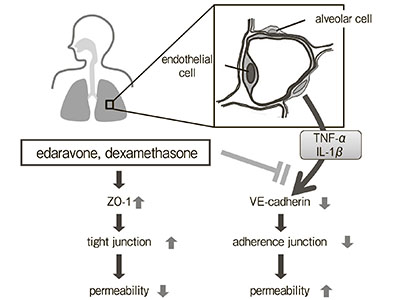
Inhibitory Effects of Edaravone, a Free Radical Scavenger, on Cytokine-induced Hyperpermeability of Human Pulmonary Microvascular Endothelial Cells: A Comparison with Dexamethasone and Nitric Oxide Synthase Inhibitor
Lung hyperpermeability affects the development of acute respiratory distress syndrome (ARDS), but therapeutic strategies for the control of microvascular permeability have not been established. We examined the effects of edaravone, dexamethasone, and N-monomethyl-L-arginine (L-NMMA) on permeability changes in human pulmonary microvascular endothelial cells (PMVEC) under a hypercytokinemic state. Human PMVEC were seeded in a Boyden […]
Experience of using methods for syndromic and pathogenetic therapy in pneumonia caused by COVID-19 at the pulmonary department
The treatment of pneumonia caused by SARS-CoV-2 virus is extremely important today. Coronavirus disease (COVID-19) is a new infection, against which there are no specific drugs or vaccines. In many ways, its complications became unexpected. Leading experts believe that vaccines and/or special drugs for the treatment of SARS-CoV-2 infection will be developed in the nearest […]
Posted: Medical newspaper "Health of Ukraine", № 13-14 (482-483), July 2020