The treatment of pneumonia caused by SARS-CoV-2 virus is extremely important today. Coronavirus disease (COVID-19) is a new infection, against which there are no specific drugs or vaccines. In many ways, its complications became unexpected. Leading experts believe that vaccines and/or special drugs for the treatment of SARS-CoV-2 infection will be developed in the nearest future. While this search continues, physicians around the world are experimenting with a variety of therapeutic modalities and existing drugs to treat patients with COVID-19.
During the pandemic period, physicians from many countries have gained extensive experience considering the course of coronavirus-associated pneumonia, its complications and causes of death. Unfortunately, there are currently no well-managed protocols for the treatment of pneumonia caused by the new coronavirus, so often doctors supplement previously established treatment regimens for viral and bacterial pneumonia with drugs that do not yet have sufficient evidential effect for the treatment of pneumonia specifically, but which may be useful due to their known pharmacological mechanisms of influence on certain links of the COVID 19 pathogenesis.
A pandemic of a new coronavirus infection that develops as a result of SARSCoV2 infection and is severely manifested by acute respiratory distress syndrome (ARDS) has caused a global crisis that is the biggest challenge to the global health system for the last 100 years. Approximately 15-20% of patients, especially the elderly ones and those suffering from serious comorbidities, have a severe form of the disease which is characterized by the 4% risk of death [7].
Penetration of SARS-CoV-2 into the cell and development of the immune response
The main target of the virus is lung epitheliocytes. It has been shown that SARSCoV2 can use the angiotensin-converting type 2 enzyme (ACE2) receptor to penetrate cells; this receptor is the same that facilitates infection of the respiratory epithelium and alveolocytes with type 2 SARSCoV virus. After binding with the SARSCoV2 virus, the latter enters the cells, followed by internalization, replication, and release of new virions from the infected cell. They affect the target organs and induce the development of local and systemic inflammatory response.
The main target of the virus is lung epitheliocytes. It has been shown that SARSCoV2 can use the angiotensin-converting type 2 enzyme (ACE2) receptor to penetrate cells; this receptor is the same that facilitates infection of the respiratory epithelium and alveolocytes with type 2 SARSCoV virus. After binding with the SARSCoV2 virus, the latter enters the cells, followed by internalization, replication, and release of new virions from the infected cell. They affect the target organs and induce the development of local and systemic inflammatory response.
It is not yet clear how the virus can avoid an immune response and stimulate pathogenesis. To overcome antiviral activity, SARSCoV encodes viral antagonists that modulate the induction of interferon (IFN) and cytokines; such mechanism allows to evade the effector function of serum immunoglobulins [8, 9].
In the human respiratory tract, SARSCoV2 causes suppression of mucociliary clearance and epithelial cell death, penetrates through the mucous membrane of the nose, larynx and bronchial tree into the peripheral blood and affects such organs as lungs, digestive tract, heart, kidneys. There are two phases of SARSCoV2 infection: early and late.
The early phase is usually manifested by a mild severity of the disease, and the main role in it is played by non-specific defense mechanisms and a specific adaptive immune response, which allow to eliminate the virus from the body. At this stage, it is recommended to carry out therapeutic measures aimed at strengthening the immune response [10]. Like many viruses, SARSCoV2 encodes proteins that counteract innate immune protection, particularly by inhibiting the activity of IFN type 1 [11].
The response of the innate immune system among infected patients is currently insufficiently studied. A key manifestation of innate immune activation during COVID19 is an increase of neutrophils amount, as well as an increase in the serum concentration of interleukin 6 (IL6) and C-reactive protein (CRP) [12]. Lymphocytopenia is developing during severe forms of COVID19 [13].
Damaged ACE2-expressing cells produce proinflammatory cytokines that recruit effector cells (macrophages, neutrophils) and release alarmines that induce inflammasome activity. Inflammasomes functioning is accompanied by the release of a significant amount of pro-inflammatory cytokines, which, in turn, enhance the recruitment of macrophages and neutrophils, and the development of the so-called cytokine storm, that causes an extraordinary level of inflammation in the lungs.
“Cytokine Storm”
“Cytokine storm” is characterized by a surge in the production of proinflammatory cytokines: IFNα and γ, IL1β, 6, 12, 18, 33, tumor necrosis factor, granulocyte-macrophage colony-stimulating factor (GMCSF), chemokines (CCL2, CCLC, CCL5, CCLC, etc.). They recruit effector immunocytes, leading to the development of a local inflammatory response. “Cytokine storm” causes ARDS development, which is accompanied by oxidative damage of cytomembranes lipids, an increase in the content of reactive oxygen species (ROS) by 10 times, and the release of lysosomal proteases.
Apoptosis of pulmonary epithelial and endothelial cells develops, cell barrier is getting damaged, vascular permeability increases, resulting in edema and hypoxia. All this leads to multiple organ failure, which can be fatal. It was found that the risk of lethal outcome of the disease is associated with high levels of IL6 in the serum [4, 14].
The consequences of high viral replication and “cytokine storm” are massive lesions of target body tissues [10]:
- vascular endothelium, which causes systemic inflammation, alteration of coagulation homeostasis, thromboembolism;
- lungs, associated with the development of pneumonia and ARDS;
- cardiovascular system, which leads to heart attacks, myocardites and death;
- intestines accompanied by diarrhea;
- kidney, associated with the development of acute renal failure;
- other organs and systems.
The release of significant amounts of cytokines is closely related to the development of known clinical symptoms of COVID19 [15]:
- IFN-γ causes fever, chills, headache, dizziness and fatigue (endogenous intoxication syndrome);
- TNF can cause flu-like symptoms (fever, malaise, weakness) and increased vascular permeability, cardiomyopathy, lung damage and acute phase protein synthesis;
- IL-6 can сause increased vascular permeability, activation of the complement system and coagulation cascade, which is associated with the specific symptoms of severe cytokine release syndrome (CRS), including disseminated intravascular coagulation (DIC); in addition, IL6 is likely to cause cardiomyopathy, followed by myocardial dysfunction, which is common among patients with CRS;
- activation of endothelial cells may also be a sign of severe CRS, and endothelial dysfunction may lead to the development of capillary leakage, hypotension and coagulopathy.
Endothelial dysfunction
Among patients with a high risk of cardiovascular disease development, decrease of ACE receptor expression in the vessel wall as a result of SARSCoV2 receptor internalisation exacerbates signs of cardiovascular pathology, endothelial dysfunction, and inflammation, especially in the presence of atherosclerosis and diabetes [16].
Endothelial dysfunction, in turn, causes the development of inflammatory processes and thrombosis, accompanied by impaired coagulation, increased fibrinogen levels, decreased fibrinolysis and anticoagulation. The production of nitric oxide (NO) is significantly reduced due to its increased destruction by the free radicals, and due to the reduced availability of the L-arginine as NO-precursor, which leads to a predominance of vasoconstrictors and increased platelet adhesion. Therefore, exogenous intake of L-arginine as a substrate for NO synthesis is pathogenetically justified by the possibility to level out endothelial dysfunction [17].
Lesions of the respiratory system
Lung damage is a major cause of both severe course and mortality due to COVID19 [18].
At the first stage of lung damage development, alveolar macrophages, having recognized SARSCoV2, begin to produce proinflammatory interleukins and chemokines, which recruit effector T-lymphocytes. In the late period of the disease development, extremely high levels of proinflammatory cytokines (IL6, 1β, TNF, etc.) production provide an influx of large numbers of monocytes and neutrophils, which exacerbate inflammation and cause pulmonary edema [10].
IL1β and TNF induce the activity of the hyaluronan synthase 2 enzyme in endothelial CD31+ cells, alveolar epithelial EpCAM+ cells of the lungs and fibroblasts, which leads to excessive production of hyaluronic acid and accumulation of fluid in the alveolar space, which in turn plays a key role in the development of inflammation and edema [19, 20].
Risk factors associated with the development of ARDS and its progression to the lethal outcome include old age, elevated neutrophil counts, organ dysfunction, and coagulopathy (e.g., higher lactate dehydrogenase activity and D-dimer content). The severity of lung damage correlates with significant pulmonary infiltration by neutrophils and macrophages and a higher number of these cells in the peripheral blood.
Neutrophils are the main source of chemokines and cytokines. They are recruited into the lungs by cytokines, which in turn become activated and release toxic mediators, accompanied by the formation of free radicals and ROS. The latter inhibit endogenous antioxidants, which leads to oxidative damage of the lung tissue cells [21].
Patients with pneumonia caused by coronavirus infection who have developed ARDS have more neutrophils than patients without ARDS. This may be due to the activation of neutrophils for the realization of the immune response against the virus, but at the same time it leads to the so-called cytokine storm [8].
The virus is thought to start a second attack, causing deterioration approximately 7-14 days after the onset of the disease. On average, 8 days pass from the appearance of the first symptoms of COVID19 to the development of ARDS [22].
The development of infection associated with the SARSCoV2 virus is accompanied by excessive activation of cellular immunity. Also patients with COVID19 have a high content of proinflammatory CCR6 + Th17 cells.
Excessive activation of Th17 cells and extremely high levels of cytotoxicity of CD8 + T cells may be responsible for the severity of immune damage within lung tissue. Moreover, patients demonstrate a depletion of the T-reg cells pool, which causes unlimited activation of inflammatory mechanisms and delays the resolution of the inflammatory process [18].
Cardiovascular system lesions
Infection with SARSCoV2 virus can inhibit ACP2 expression activity, leading to toxic over-accumulation of angiotensin II, which causes ARDS and myocarditis [23].
Myocardial damage associated with SARSCoV2 virus infection, accompanied by a sharp increase in troponin I concentration (> 28 pg/ml), was observed in 5 of the first 41 patients diagnosed with COVID19 in Wuhan, China [24].
More than 60% of patients with lethal outcome because of COVID19, had hypertension, cardiovascular pathologies or diabetes in the anamnesis [25].
It has been suggested that blockade of the renin-angiotensin system increases ACE 2 expression, leading to internalization of SARSCoV2 into lung and heart cells, which cause ARDS, myocarditis, and death [26].
Other possible mechanisms of myocardial damage include a “cytokine storm” caused by an imbalance of the Th1 and T-reg cells response, and hypoxemia caused by COVID19 [27].
Hypercoagulation and Thrombosis
Most critical patients have severe systemic disease and multiple organ failure in addition to respiratory symptoms (pneumonia and ARDS). This is why many patients with COVID19 do not respond adequately to provided respiratory support.
One of the most significant manifestations associated with poor prognosis is the development of coagulopathy and endothelial dysfunction with diffuse micro- and macrothrombosis. The lungs are the most frequently affected organ due to the highest level of inflammation.
This justifies the idea that patients should not be transferred to artificial lung ventilation (ALV) too early, because without adequate blood flow inside the lungs such approach is useless. The main idea of ventilation is to improve oxygenation, which requires an optimal ratio of ventilation and perfusion, as well as maintaining the mechanism of venous hypoxic vasoconstriction. In fact, 9 out of 10 patients die because of cardiovascular rather than respiratory causes, since venous microthrombosis, and not pneumonia, determines mortality, and thrombosis and endothelial dysfunction are the triggers of respiratory failure among a significant amount of patients [8].
A number of reports indicate a high incidence of both arterial and venous thromboembolic complications among patients with COVID19, suggesting the need for the use of simple and accessible laboratory markers. The incidence of venous thromboembolism can be as high as 25%, sometimes resulting in lethal outcome. Patients have an increased incidence of heart attacks, strokes and other thrombotic diseases.
Among hematological changes, an increase in the content of D dimer, fibrinogen and other inflammatory markers is noted. In contrast to classical DIC, these patients have a lower degree of increase in activated partial thromboplastin time in comparison to prolongation of prothrombin time (probably due to elevated levels of VIII factor). DIC may develop in later stages of the disease, causing a prognosis worsening. Anti-inflammatory drugs should be used due to the critical role of thromboinflammation and endothelial dysfunction during the conditions of “cytokine storm” in the early stages of the disease [28].
Treatment approaches of COVID19 patients and its complications
Since generally accepted approved treatment method for a new coronavirus infection is absent, specialists are forced to use drugs that are not registered as medicinal products for the treatment of COVID19 to alleviate the patient symptoms.
Thus, a situation has occurred, in which practical clinical experience becomes more important than the principles of evidence-based medicine [29].
Due to the fact that there is currently no etiotropic therapy with proven efficacy, treatment approaches of COVID19 patients should include pathogenetic, symptomatic and replacement therapy.
The World Health Organization’s guideline from March 13, 2020 states that treatment of mild COVID19 form should include symptomatic therapy and monitoring; the severe form of pathology requires oxygen therapy, monitoring and treatment of coinfection. Critically ill patients require additional treatment measures for ARDS and septic shock and the prevention of complications [1].
About 15% of adults infected by SARSCoV2 suffer from severe pneumonia, which requires additional oxygen insufflation. In 5% of patients, the course of pneumonia progresses to a critical level with the development of hypoxemic respiratory failure, ARDS and multiple organ failure, requiring respiratory support often during a few weeks [30].
Pneumonia caused by the COVID19 is characterized by an increase of effector T-cells, inflammatory cytokines and D-dimer, activation of the coagulation cascade, increased levels of fibrosis and microembolism, which must be taken into account when choosing a therapeutic strategy.
As life-threatening systemic and pulmonary inflammation develops rapidly, early detection of clinical and humoral markers with reactive hyperimmune response becomes important. Therefore, early initiation of treatment can dramatically affect the therapy effectiveness and minimize the possible impact of viral replication [31, 32].
Syndrome-pathogenetic approach
When choosing a treatment regimen for patients with COVID19, it is important to keep in mind the need of mitigation of consequences in order to reduce the frequency of disablement status development and avoid a decline in quality of life for those who have suffered from this serious disease. Among the possible consequences, the following should be noted:
- primarily somatic disorders: microthromboendotheliitis, which is a systemic vascular damage with the formation of microthrombi, resulting in the respiratory, excretory, cardiovascular, endocrine, gastrointestinal systems alterations; fibrous changes in lung tissue, pneumosclerosis. Lung patency restoration could be obtained by medication supply (broncho- and mucolytics, steroids), intrapulmonary percussion ventilation. The therapy of patients with diabetes mellitus, whose vessels, especially small ones, have already been affected, should be adjusted with sugar-lowering drugs;
- consequences of treatment with the use of dangerous drugs: gastrointestinal complications associated with COVID19 are mainly presented by hepatic insufficiency, and largely related to drug exposure;
- exacerbation of chronic diseases, changes in the nature of their course, which requires correction;
- psychological problems, depression development. The formation of the rehabilitation measures complex should be based on the specific clinical situation. The application of the syndrome-pathogenetic approach which consists of specific manifestations and syndromes treatment, instead of diagnosis treatment, acquires extreme urgency.
For example, when the endothelium of the lungs is getting affected, the plasma is moved into the interstitial space, then hyaline membranes are formed, and this is how fibrosis process is developing. However, even if fibrotic changes persist for a long time, a syndromic approach should be followed: oxygen support and non-invasive lung ventilation should be provided only in case of respiratory failure. Moreover, the use of lung ventilators in the case of COVID19 is associated with high (up to 90%) mortality, ventilator-associated pneumonia and other complications [33].
Methods of COVID19 pharmacotherapy
Among the drugs used for the COVID19 control there are antiviral drugs (both for monotherapy and for various combinations, including interferons). For example, nelfinavir developed for the combination treatment of HIV patients, is included in the list of potentially effective drugs for the COVID19 treatment [34].
There are also data on the effectiveness of remdesivir, which acts directly on the causative agent of coronavirus infection: its use helped to improve the condition of 68% of patients, reducing the length of hospital stay from 15 to 11 days [35].
Favipiravir, which was previously used for influenza treatment, and is now successfully implemented as an experimental COVID19 treatment, acts on one of the viral RNA replication units, halving the incidence of fatalities and reducing the incidence of serious complications in 4 times [36, 37].
Data considering the use of chloroquine-based drugs (sometimes in combination with the antibiotic azithromycin) among patients with COVID19 is contradictory: these agents provide antiviral effects in high concentrations, leading to toxical influence; in addition, cardiac arrhythmia is a serious side effect of their use [38].
The use of recombinant human monoclonal antibodies to IL6 receptors, such as levilimab and tocilizumab, as well as other immunosuppressive agents that prevent an overactive immune response that could cause worsening of the clinical condition is of particular interest. Thus, tocilizumab blocks IL6 receptors, which contributes to a positive therapeutic effect during many inflammatory diseases, including COVID19 [39].
A retrospective study of the tocilizumab efficacy during patients treatment with severe or critical COVID19 condition has shown that this drug improved oxygenation rate among 75% of the patients and normalized peripheral lymphocyte counts among 52,6% of patients [40].
Patients with weakened immune system may be more prone to complications of COVID19 due to the lack of a rapid immunological response that helps to overcome the virus. However, it has now been suggested that immunosuppression may also play a protective role during COVID19 infection, preventing an overactive immune response, which sometimes worsens or weakens the clinical condition of the patient [41].
In particular, a case of B-cell depletion was described, during which COVID19 developed without serious complications in patient suffering from multiple sclerosis, bronchial asthma and peptic ulcer disease. The authors of the publication suggested that the persistence of B-cells in secondary lymphoid organs, associated with a moderately reduced immune response due to the absence of peripheral cells, may have played a favorable role for the clinical outcome. The fact that the patient did not show significant increase of IL6 amount (which may be released by peripheral B-cells) probably supports this hypothesis. If such hypothesis will be confirmed in more cases, it can be assumed that patients who experience B-cell depletion are protected from serious complications of COVID19. This would favor the use of selective immunosuppressants during severe COVID19 cases [42].
Since the activity of the hyaluronan synthase 2 enzyme in the lung tissues leads to an excess of hyaluronic acid production, it is thought that inhibition of its production will increase the area of gas exchange surface in the alveoli and support the recovery of COVID19 patients.
In particular, it is proposed to use for such purpose hymecromone as an approved drug for the treatment of gallbladder dysfunction, which is an inhibitor of hyaluronan synthase 2 [13].
According to recent clinical reports, the therapeutic range during the COVID19 is significantly longer than 14 days, but prolonged exposure to the virus can lead to the development of sudden intense immunological reactions, “cytokine storm” and immune cells infiltration. Some immunocytes, especially macrophages and neutrophils, are able to produce numerous ROS [43, 44].
A certain level of ROS is important for the regulation of immune responses and for clearance from viruses, but their excessive content leads to oxidation of cellular proteins and membrane lipids, as well as to rapid destruction of not only virus-infected but also normal lung cells and even heart cells, as a result of which multiorgan failure develops.
Therefore, antioxidant therapy can be proposed as a potential therapeutic approach for COVID19-induced lung and cardiovascular tissue damage reduction. Potential drugs include antioxidants such as vitamin C (ascorbic acid) and vitamin E, since their reducing hydrogen atoms can react with ROS and neutralize them without the formation of toxic substances. Also molecules of plant origin (used in ancient Chinese medicine), such as curcumin and baikalin should be mentioned [45].
It is suggested that an adequate dose of antioxidants may help to reduce heart and lung damage in patients with severe COVID19 form, but scientists do not rule out that this approach may be also useful for patients with mild symptoms of disease [46].
Possibilities of edaravon, L-arginine, L-carnitine and Reosorbilact use in complex treatment of patients with COVID-19
The potential benefits of antioxidant therapy depend on the right choice of drugs. The key requirements for them are the ability to affect mitochondrial permeability, block the signaling pathways of inflammation (IL1, 6, 18) and act in synergy with water-soluble antioxidants (in the cytoplasm).
Edaravone
Edaravon is a low-molecular-weight antioxidant drug that demonstrates targeted interaction with peroxyl radicals. Due to its amphiphilicity, it absorbs both fat and water-soluble peroxyl radicals by transferring an electron to the radical; inhibits the oxidation of lipids by absorbing water-soluble peroxyl radicals that initiate chain chemical reactions, as well as fat-soluble peroxyl radicals that support this chain [47].
In 2001, this drug was approved in Japan as a drug for the treatment of acute ischemic stroke, and in 2015 it was approved for the treatment of amyotrophic lateral sclerosis. Recommendations of the Japanese societies considering the use of respiratory support and intensive care include edaravon as a drug that should be used for the treatment of patients with ARDS within intensive care units [52]. Edaravon is able to quickly neutralize a wide range of free radicals. It activates eNOS and can improve blood circulation, blocks inflammatory iNOS; effectively inhibits lipid peroxidation (LPO), and protects cells from destruction by suppressing the chain reaction of LPO by absorbing peroxyl radicals.
Edaravon activates the enzymes of antioxidant protection, such as SOD (superoxide dismutase), CAT (catalase) and GSHRx (glutathione peroxidase).
The drug easily penetrates the blood-brain barrier, unlike other free radical acceptors, which explains its pronounced therapeutic effect; protects the endothelium of the brain from damage due to the protective effect on microvessels, which determines its possible therapeutic effect during disorders and diseases associated with carbonyl stress [48]. Edaravon reduces damage of the blood-brain barrier and inhibits the development of cerebral edema, directly and indirectly reduces the production of such pro-inflammatory cytokines, as IL6, iNOS, TNF and metalloproteinases.
Edaravon has been used for almost 20 years for treatment of the stroke patients (for the first 24 hours), but due to its free radical scavenger properties, it has a protective effect on the heart, lungs, intestines, liver, pancreas, kidneys and other organs. Edaravon has recently been shown to be useful in patients with acute myocardial infarction. The drug also helped to increase the fraction of left ventricular ejection and reduce the frequency of re-hospitalizations among patients with acute myocardial infarction. Animal studies have shown that edaravon reduced the number of IL1β-positive myocardial cells among rats with experimental autoimmune myocarditis; protected cardiac function and reduced the size of the heart attack by decreasing the production of TNF in the myocardium [49].
Experimental studies have shown that edaravon is able to prevent the development of increased permeability of endothelial cells in the pulmonary microcirculatory tract caused by proinflammatory cytokines, probably due to increased adhesive contacts, as well as due to other mechanisms of action. Thus, the drug may be useful for the treatment of patients with ARDS and pneumonia in clinical practice. The safety of edaravon has been proven in a number of studies [50, 51].
Thus, the use of edaravon, which is capable of effectively “extinguishing a cytokine fire,” can significantly improve the condition of patients with COVID19, the pathogenesis of which is associated with the development of ARDS and a sharp increase of cytokine levels.
L-arginine
L-arginine is a conditionally essential amino acid that is an active cellular regulator of many vital functions of the body, revealing protective effects that are important in a critical condition. Arginine has antihypoxic, membrane stabilizing, cytoprotective, antioxidant, antiradical and detoxifying effects.
This amino acid is a substrate for the synthesis of NO, and because of that it improves microcirculation, promotes strong vasodilation, prevents activation and adhesion of lymphocytes and platelets.
The physiological action of NO varies from the modulation of the vascular system to the regulation of immune processes (cell-mediated immunity, the effect of neutrophils on pathogenic microorganisms, nonspecific immune protection) and the control of neuronal functions.
L-arginine restores the content of NO in the lung tissue, which reduces the spasm of the unstriated muscles of the bronchi and improves the vasomotor function of the endothelium among the pulmonary arteries [53]. L-arginine is effective for blood pressure decrease among patients with hypertension [54]. With COVID19 vaccine under development and no universal effective treatment available, the next question arises: how can the avoidance of the pathology be ensured? It is especially relevant for the elderly and medically compromised patients.
Based on experts opinion, the use of drugs that contribute to the overall strengthening of the body and improve the protection function of the immune system is promising. Such substances, of course, include L-arginine [55].
The usefulness of this approach is confirmed by a clinical trial initiated by the Medical Center of Ohio State University, USA, dedicated to the use of inhaled NO for the treatment of patients diagnosed with COVID19. The goal is to avoid intensive care [56].
Therefore, increasing the NO content is a promising perspective of treatment for patients with coronavirus infection, and L-arginine can be successfully used to achieve this goal.
L-carnitine
L-carnitine is a natural substance involved in energy metabolism as well as the metabolism of ketone bodies. It is necessary for the transportation of long-chain fatty acids into the mitochondria (which are used as an energy substrate by all tissues except the brain) for their further oxidation and energy production.
This substance plays an important role in cardiac metabolism, as the oxidation of fatty acids depends on the availability of its sufficient amounts. The positive effect of L-carnitine has been proved during acute and chronic ischemia, decompensation of cardiac activity, heart failure caused by myocarditis and drug cardiotoxicity.
The immunomodulatory effect of L-carnitine is based on the inhibition of proinflammatory cytokines TNF, IL6 and IL1 under the conditions of “cytokine storm”. The drug is a direct antioxidant that prevents cell apoptosis and has a cardioprotective effect.
The results of two major meta-analyzes provided by DiNicolantonio (2013) and Askarpour et al. (2019) confirmed the effectiveness of L-carnitine therapy in patients with cardiovascular disease by reducing mortality, improving quality of life, lowering cholesterol level, normalizing heart rate and reducing the need for nitrates intake [57, 58].
L-carnitine may be beneficial for patients with COVID19 due to its immunomodulatory effects and inhibition of inflammatory mediators, including CRP, TNF and IL6, which may help to reduce the “cytokine storm” [59].
Reosorbilact
Reosorbilact is a unique hyperosmolar crystalloid solution that does not contain excess of chlorine, and therefore helps to avoid the main disadvantage of such solutions, such as an increased risk of hyperchloremia.
An important feature of Reosorbilact is the combination of both hyperosmolar properties and the properties of balanced crystalloids (a set of necessary Ca2 +, K +, Na +, Mg2 + ions in isoplasmic concentration). Due to hyperosmolarity, Reosorbilact induces fluid transfer from the intercellular sector to the vascular bed, which improves microcirculation and tissue perfusion due to the potent osmodiuretic effect of sorbitol, which is associated with the lack of natural human mechanisms of reabsorption for polyhydric alcohols in renal proximal tubules. Reosorbilact has a pronounced diuretic and anti-edematous effect [60].
The main pharmacologically active ingredients of the drug are sorbitol and sodium lactate. Sorbitol improves microcirculation and tissue perfusion, and has a disaggregating effect.
Sodium lactate helps to correct metabolic acidosis; sodium chloride has a rehydrating effect, and replenishes the deficiency of sodium and chlorine ions in various pathological conditions; calcium chloride restores the content of calcium ions necessary for the transmission of nerve impulses, contraction of skeletal and non-striated muscles, myocardial activity, bone formation, blood clotting, and prevents the development of inflammatory reactions, increases the body’s resistance to infections; potassium chloride restores water-electrolyte balance, has a negative chronotropic and bathmotropic effect, and moderate diuretic effect. Several studies have shown that the use of hypertonic saline in patients with hemorrhagic shock due to traumatic injury reduces the length of stay on mechanical ventilation
It has also been found that the use of hyperosmolar solutions during the treatment of patients with severe sepsis can reduce the volume of infusion. Such positive effects as reduction of pulmonary complications, duration of mechanical ventilation and volume of infusion are important and promising during the use of hyperosmolar solutions for patients with pneumonia, particularly caused by COVID19, when it is important to adhere to a restrictive infusion [61, 62].
Study objective
In view of the foregoing, we conducted a study on the effectiveness of the syndrome-pathogenetic treatment approach. The objective was to evaluate the effect of combination including edaravon (Xavron), L-arginine hydrochloride + L-carnitine (Tivorel) and Reosorbilact (sorbitol, sodium lactate, sodium chloride, calcium chloride, potassium chloride, magnesium chloride) on clinical course of moderate severity pneumonia, caused by COVID19, as adjunctive therapy to basical treatment under conditions of a pulmonary department.
Design of the study
The study included two groups of 30 patients each with diagnosed moderate-grade pneumonia caused by COVID19. The main group received a combination of edaravon (Xavron) 30 mg 2 times per day intravenously, Larginine hydrochloride + L-carnitine (Tivorel) 1 vial / day intravenously and Reosorbilact 200 ml per day in addition to basic therapy. The control group received basic therapy.
Control points
The studied indicators were: the number of days spent in the hospital, saturation (oxygen saturation of the blood), body temperature, the levels of ferritin, D-dimer, CRP, procalcitonin; general clinical blood test to determine the number of erythrocytes, leukocytes, platelets, ESR, leukocyte formula and hemoglobin content. Patients also underwent computed tomography of the chest area.
Biochemical analysis of blood does not provide specific information, but the detected abnormalities may indicate the presence of organ dysfunction, decompensation of comorbidities and the development of complications; so such changes have some prognostic value, indicating the effectiveness of the drugs choice.
Ferritin content. Hyperferritinemia is called a marker of severe coronavirus infection associated with a high risk of “cytokine storm” development. It is noted that researchers around the world are looking for a way to quickly reduce the level of ferritin in the blood of such patients.
The content of D-dimer, which is 3-4 times higher compared to the age norm, has clinical significance.
The content of CRP is the main laboratory marker for the activity of the process going on in the lungs; its increase correlates with the extent of lung tissue damage and is the basis for starting anti-inflammatory therapy.
Lymphocytopenia and thrombocytopenia. Leukocyte counts are normal in most patients with COVID-19; one third have leukopenia, and 83.2% have lymphopenia; thrombocytopenia is moderate, but is more common in patients with severe form of disease.
Laboratory signs of “cytokine storm” and ARDS may be following: a sudden increase in clinical manifestations after 12 weeks from the onset of the disease; febrile fever that increases or reappears; severe lymphopenia in complete blood count; reduction of the T- and B-lymphocytes number; a significant increase of the D-dimer level (> 1500) or its rapid increase; increase of CRP content> 75 mg/l.
The development of cardiovascular complications in the case of COVID19 is also accompanied by lymphocytopenia, thrombocytopenia and increased CRP level.
Results and Discussion Figure 1 demonstrates the mean values of CRP (mg/l) before and after treatment among patients of the main and control groups. If before treatment the difference between the values was statistically insignificant (39.45 ± 9.7 vs. 46.26 ± 5.53 mg/ml, respectively), then after treatment patients of the main group demonstrated significant improvement compared to the patients in the control group (7.59 ± 1.71 vs. 12.5 ± 1.67 mg/ml, respectively; p = 0.04).
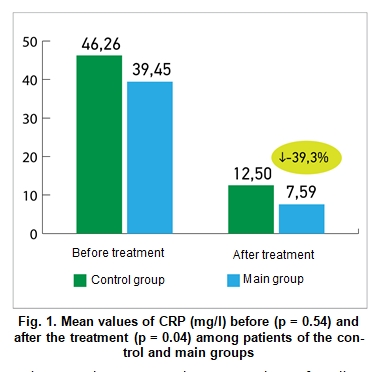
Figure 2 demonstrates the mean values of D-dimer (ng/ml) before and after treatment among patients of the main and control groups. The difference between the D-dimer levels was statistically insignificant before treatment (1304.54 ± 230.32 vs. 1541.06 ± 477.79 ng/ml, respectively), but after treatment patients of the main group demonstrated significant improvement compared to the patients of the control group (1445.38 ± 106.03 vs. 1903.27 ± 129.68 ng/ml; p = 0.01).
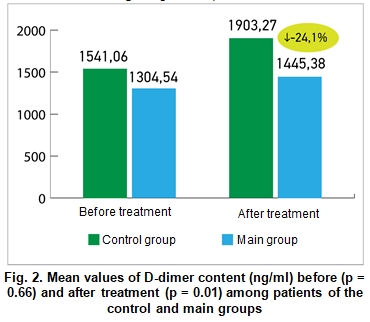
Figure 3 demonstrates the mean values of ferritin content (ng/ml) before and after treatment among patients of the main and control groups. The difference between the ferritin values was statistically insignificant before the treatment, (500.6 ± 89.14 vs. 598 ± 94.03 ng / ml, respectively), but after the treatment patients of the main group demonstrated significant improvements compared to the patients from the control group (393.72 ± 51, 73 vs. 540.11 ± 49.93 ng/ml, respectively; p = 0.05).
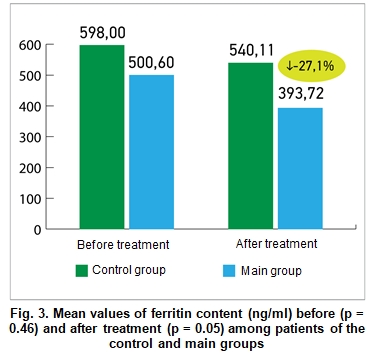
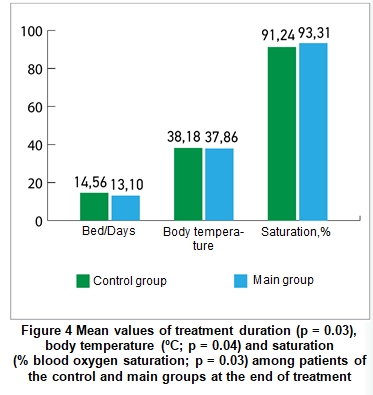
Figure 4 makes it possible to compare the average values of treatment duration, body temperature and saturation among patients of the main and control groups. All indicators at the end of treatment were significantly better in patients of the main group (combination of basic therapy with additional treatment with Xavron, Tivorel and Reosorbilakt) compared to the patients in the control group: saturation levels were 93.31 ± 0.57 vs. 91.24 ± 0.77 (%oxygen saturation; p = 0.03), body temperature values were 37.86 ± 0.1 vs. 38.18 ± 0.12 ( oC, p = 0.04), the number of bed/days in the hospital was 13.1 ± 0.49 vs. 14.56 ± 0.42 (p = 0.03), respectively.
Conclusions
• The results of the study have shown that a number of indicators in the main group (patients whose basic treatment of pneumonia caused by COVID19 was combined with adjunctive therapy by Xavron, Tivorel and Reosorbilact) has significantly improved compared with the control group (patients who received only basic treatment). Adjunctive therapy helped to improve blood oxygen saturation, lower body temperature, and reduce hospital duration stays.
• At the end of treatment the main group demonstrated significantly decrease of CRP concentration, which is a marker of inflammatory activity in the lungs, and increase of which correlates with the extent of lung tissue damage and the severity of the disease.
• A decrease of the D-dimer content, which also belongs to the markers of the inflammatory process, among patients of the main group indicates an improvement considering their condition compared to the control group.
• Increase of acute phase ferritin protein concentration was noted in case of an unfavorable course of a disease and during so-called cytokine storm, so decrease of this specific indicator in the main group demonstrated an evidence of adjunctive therapy effectiveness using Xavron, Tivorel and Reosorbilact.
• Special attention is now being paid to the application of a syndrome-pathogenetic approach of coronavirus infection treatment, since etiotropic drugs that could affect directly COVID19 pathogen have not yet been developed. Considering such conditions, it is extremely important to use a comprehensive approach during the treatment of patients with severe forms of disease, which takes into account individual characteristics and provide the most effective support for the body. Every effort should also be made to prevent disablement status development and reduction of patients quality of life after illness due to possible complications (damage of the respiratory system due to fibrosis, severe consequences for the cardiovascular system due to thrombosis, etc.). This will be facilitated by the additional use of Xavron, Tivorel and Reosorbilact.
Perspectives of further research
It was established that, in addition to pneumonia, COVID19 is accompanied by the development of respiratory failure, changes in rheological and fibrinolytic properties of blood, increased thrombosis, damage to the cardiovascular and nervous systems, and increased fibrosis in the lungs. The tendency of fibrosis manifestations decrease was noted in the experimental group during the research. Therefore, it would be useful to investigate the effect of our proposed combination of drugs on the fibrosis processes within the lungs after viral pneumonia, given the wide range of already known therapeutic properties of these drugs.
Author: S.V. Kovalenko, Doctor of Medical Sciences, Department of Internal Medicine, Clinical Pharmacology and Professional Diseases Bukovinian State Medical University; Pulmonary Department of Chernivtsi Regional Clinical Hospital.
Posted: Medical newspaper "Health of Ukraine", № 13-14 (482-483), July 2020