Lung hyperpermeability affects the development of acute respiratory distress syndrome (ARDS), but therapeutic strategies for the control of microvascular permeability have not been established. We examined the effects of edaravone, dexamethasone, and N-monomethyl-L-arginine (L-NMMA) on permeability changes in human pulmonary microvascular endothelial cells (PMVEC) under a hypercytokinemic state. Human PMVEC were seeded in a Boyden chamber. After monolayer confluence was achieved, the culture media were replaced respectively by culture media containing edaravone, dexamethasone, and L-NMMA. After 24-h incubation, the monolayer was stimulated with tumor necrosis factor-α(TNF-α) and interleukin-1β(IL-1β). Fluorescein-labeled dextran was added. Then the transhuman PMVEC leak was measured. Expressions of vascular endothelial-cadherin (VE-cadherin) and zonula occludens-1 protein (ZO-1) were evaluated using real-time quantitative polymerase chain reaction and immunofluorescence microscopy. The results showed that TNF-α+IL-1βmarkedly increased pulmonary microvascular permeability. Pretreatment with edaravone, dexamethasone, or L-NMMA attenuated the hyperpermeability and inhibited the cytokine-induced reduction of VE-cadherin expression on immunofluorescence staining. Edaravone and dexamethasone increased the expression of ZO-1 at both the mRNA and protein levels. Edaravone and dexamethasone inhibited the permeability changes of human PMVEC, at least partly through an enhancement of VE-cadherin. Collectively, these results suggest a potential therapeutic approach for intervention in patients with ARDS.
Influenza virus infection causes severe pneumonia, especially in children and adults with underlying disease [1, 2]. Many patients need ventilator support because of severe complications such as acute respiratory distress syndrome (ARDS) [3]. Influenza A/H1N1pandemic viruses have been reported to show an affinity for type II alveolar epithelial cells and to invade the lung directly [4]. Although lung hyperpermeability is thought to contribute to ARDS development [5], no clinically available medication for the control of vascular permeability has been established.
Vascular endothelial cell-cell adhesions that control vascular permeability are organized mainly by adherence junctions and tight junctions [6, 7]. Adherence junctions are formed by members of the cadherin family. Vascular endothelial-cadherin (VE-cadherin) receives positive and negative control by various signals. It regulates endothelial cell adhesion and barrier function of blood vessels dynamically [6, 8]. Tight junctions include transmembrane molecules such as occludin, claudin, and junctional adhesion molecules. Zonula occludens-1 protein (ZO-1), a lining protein of tight junctions, connects transmembrane molecules with the actin cytoskeleton. In response to inflammatory cytokines, the vascular endothelium expresses cellular adhesion molecules including intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1), and secretes chemokines such as monocyte chemoattractant protein-1 (MCP-1) and interleukin-8 (IL-8) to upregulate leukocyte recruitment into inflamed tissues [9].
Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), which is used to treat cerebral infarction as a free radical scavenger [10], reportedly protects various organs [11]. Several animal studies have demonstrated that edaravone prevents lung injury induced by various factors, including anticancer drugs, lipopolysaccharide, and acute pancreatitis via antioxidant and anti-inflammatory effects [12-15]. Nevertheless, little is known about whether edaravone has any protective effect for ARDS characterized by enhanced vascular permeability.
The 2009 pandemic influenza A virus infection caused severe pneumonia and ARDS related to lung hyperpermeability more often than seasonal influenza virus infection did [2, 3]. Although we initially tried to perform influenza virus infection experiments using human pulmonary microvascular endothelial cells (PMVEC), neither influenza A/H1N1pandemic nor A/H3N3Aichi was found to infect endothelial cells or change the permeability parameters (unpublished data). Therefore, we specifically examined the permeability changes of endothelial cells by inflammatory cytokines that are produced during severe infectious diseases including influenza. For this study, we established a human PMVEC monolayer model to examine the barrier permeability changes that occur in endothelial cells in a hypercytokinemic state. Dexamethasone and nitric oxide synthase (NOS) inhibitor are reported to decrease pulmonary vascular hyperpermeability in ARDS [16, 17]. Therefore, we examined the therapeutic effects of edaravone on permeability changes and, in the same experimental setting, compared the effects of edaravone to those of dexamethasone and NOS inhibitor.
Cell culture. Human PMVEC were purchased from Lonza Group, Ltd. (Walkersville, MD, USA) and were maintained using a BulletKit (EGM-2MV; Lonza) as recommended by the manufacturer. The cells were cultured in 95オ air, 5オ CO2 at 37℃. All experiments were performed on cells in the 3rd to 6th passage. Permeability assay. A Boyden chamber was used for the permeability evaluation. Human PMVEC were seeded in Transwell Inserts (3.0µm pore; Becton, Dickinson and Co., Franklin Lakes, NJ, USA) at a concentration of 5×104 cells/well. They were cultured for 3 days. After monolayer confluence was achieved, the culture media in the upper chamber (150µl) and the lower chamber (750µl) were replaced respectively by culture media containing edaravone (100µM; Mitsubishi Tanabe Pharma Corp., Tokyo, Japan), dexamethasone (100 µM; Calbiochem Novabiochem Corp., La Jolla, CA, USA), and N-monomethyl-L-arginine (L-NMMA, 1 mM; Calbiochem Novabiochem Corp.). After 24h incubation, the culture medium was replaced with culture medium containing each chemical and cytokines. Tumor necrosis factor-α (TNF-α) (Peprotech Inc., Rocky Hill, NJ, USA) and interleukin-1β (IL-1β) (Peprotech Inc. ) were used as pro-inflammatory cytokines. In a preliminary set of experiments, human PMVEC were treated with TNF-α (100ng/ml) and IL-1β (100ng/ml) singly or in combination. Used alone, TNF-αand IL-1βeach tended to increase the endothelial permeability, but the permeability changes were not significant. Only the combination of TNF-α +IL-1β increased the permeability significantly (data not shown). In subsequent experiments, therefore, human PMVEC were treated with TNF-α+ 280 Saito et al. Acta Med. Okayama Vol. 69, No. 5 IL-1β at either of 2 concentrations (10ng/ml or 100ng/ml) for 24h.
Phosphate-buffered saline was used as a control buffer. To measure trans-human PMVEC dextran leak, 150µl assay medium containing fluorescein isothiocyanate-labeled dextran (FITC-Dx; MW 3,000) (100µg/ml) was added to each upper chamber; and 750µl assay medium was added to each lower chamber. After incubation for 2h at 37℃, the fluorescence intensity of medium from the lower chambers was measured at 485-538nm. The data were expressed as follows: permeability index (オ)=[(experimental clearance)-(spontaneous clearance)]×100/[(clearance of filter alone)-(spontaneous clearance)] [18]. The experiments were repeated 3 times, with each repetition consisting of 4 replicates.
Lactate dehydrogenase (LDH) release assay. Cell damage was assessed using the LDH release assay (LDH Cytotoxicity Detection Kit; Takara Bio Inc., Shiga, Japan). The percentage cytotoxicity was calculated as follows: cytotoxicity (オ)=[experimental LDH release (OD492)-spontaneous LDH release (OD492)]×100/[maximum LDH release (OD492)]- [spontaneous LDH release (OD492)]. The OD492 of spontaneous LDH release and the OD492 of maximum LDH release were obtained respectively from the supernatant of controls and the supernatant of the cells treated with 2オ Triton X-100. The experiments were repeated twice, with each repetition consisting of 4 replicates.
Immunofluorescence microscopy. For immunofluorescence experiments, the endothelial cells were grown on collagen I- coated slide chambers (Becton, Dickinson and Co.). They were subsequently treated for 24h with edaravone, dexamethasone, or L-NMMA followed by treatment with TNF-α+ IL-1β (10ng/ml) for 24h. The cells were then washed with phosphate-buffered saline and fixed in 2オ formaldehyde. After permeabilization with 0.1オ Triton X-100 and blocking, the cells were incubated with anti-VE-cadherin antibody (10µg/ml; R & D Systems, Minneapolis, MN, USA) and anti-ZO-1 antibody (5µg/ml; Genetex Inc., Irvine, CA, USA) for 60min. Alexa Fluor 488-conjugated antibody (Invitrogen Corp., Carlsbad, CA, USA) was used as a secondary antibody. The nucleus was stained with 4ʼ, 6-diamidino-2-phenylindole (DAPI) in mounting medium (Vectashield; Vector Laboratories Inc., Burlingame, CA, USA). Samples were evaluated under a fluorescence microscope (BZ-9000 generation II; Keyence Co., Osaka, Japan). The experiments were repeated 4 times.
Real-time quantitative polymerase chain reaction (real-time PCR). To examine the effects of cytokines and medications on VE-cadherin and ZO-1 mRNA expression in human PMVEC under the experimental conditions employed, real-time PCR was performed with total RNAs extracted from the cells using the specific primers presented in Table 1 [19]. After the cells reached confluence in 24-well plates, they were incubated for 24h with edaravone, dexamethasone, or L-NMMA, followed by treatment with TNF-α+IL-1β. After stimulation, the complementary DNA was extracted using FastLane® Cell cDNA (Qiagen Inc., Hilden, Germany). Real-time PCR was performed using a real-time PCR system (7500 Fast; Applied Biosystems, Foster City, CA, USA) with SYBR ®Premix Ex Taq (Takara Bio Inc.). The PCR mixture, which had a total volume of 50µl, consisted of 1×SYBR ®Premix Ex Taq, which included DNA polymerase, SYBR Green dye, dNTP mixture, and PCR buffer, 0.2µM each of the forward and reverse primers, and cDNA of the samples. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
Enzyme-linked immunosorbent assay (ELISA). Expressions of adhesion molecules in the culture supernatant were measured following Lehleʼs in vitro model [20-22]. The levels of soluble ICAM-1 (sICAM-1) and soluble VCAM-1 (sVCAM-1) were measured (Luminex 100/200 System; Luminex Corp., Austin, TX, USA) with a Human CVD Panel 1 96-well Plate Assay (Millipore Corp., Bedford, MA, USA). The levels of MCP-1 and IL-8 were also measured by ELISA (R & D Systems). The results were obtained under 2 experimental conditions, each consisting of 4 replicates.
Statistical analyses. All values were expressed as the mean ± SD. Differences between groups were examined for statistical significance using one-way analysis of variance with Tukeyʼs multiple comparison test. Statistical significance was inferred for P-values less than 0.05.
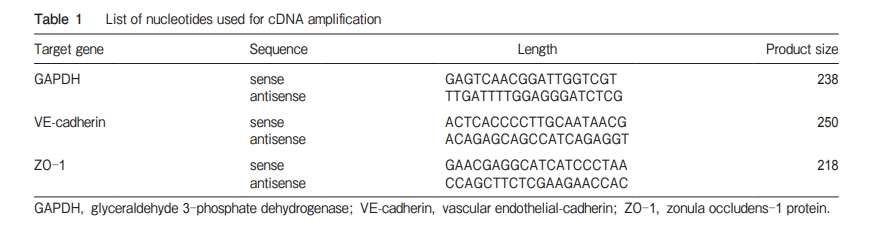
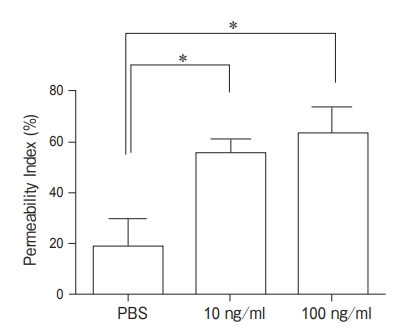
Permeability change of the human PMVEC monolayer. As presented in Fig. 1, the endothelial permeability was increased significantly by TNF-α+IL-1β at the concentrations of 10ng/ml and 100ng/ml compared to the control group. No significant difference was found between 10ng/ml and 100ng/ml. For subsequent experiments, the concentration of 10ng/ml was adopted. Pretreatment with edaravone (100µM) significantly decreased the permeability by 45オ compared with the group treated with TNF-α+IL-1βalone (Fig. 2A). Pretreatment with dexamethasone (100µM) significantly decreased the permeability by 35オ compared with the group treated with TNF-α+IL-1βalone (Fig. 2B). Pretreatment with L-NMMA (1mM) significantly decreased the permeability by 50オ compared with the group exposed to only TNF-α+IL-1β(Fig. 2C).
Cell cytotoxicity. Cell damage was assessed by using an LDH release assay. The LDH release was not altered significantly by the pretreatment of endothelial cells with edaravone, dexamethasone, or L-NMMA compared with TNF-α+IL-1β alone (Fig. 3). This result showed that the attenuation of hyperpermeability by pretreatment in this study was not caused by the inhibition of cell membrane damage or cell death. Effects of edaravone, dexamethasone, and L-NMMA on the expression of endothelial adherence junction protein VE-cadherin. The effects of edaravone, dexamethasone, and L-NMMA on the expression of VE-cadherin were examined to identify the relation between permeability changes and cell-cell adhesion. Immunofluorescence staining showed that staining for VE-cadherin was reduced after TNF-α+IL-1βstimulation. Pretreatment with edaravone, dexamethasone, or L-NMMA for 24h attenuated the effects of TNF-α+IL-1βstimulation (Figs. 4A-4E). Pretreatment with edaravone, dexamethasone, or L-NMMA for 24h followed by treatment with TNF-α+IL-1β for 3h did not alter the mRNA level of VE-cadherin (Fig. 4F).
Effects of edaravone, dexamethasone, and L-NMMA on the expression of endothelial tight junction protein ZO-1. No change of ZO-1 was shown by treatment with TNF-α+IL-1β for 24h. The near-circumferential expression of ZO-1 was increased by pretreatment with edaravone or dexamethasone for 24h compared with the other groups (Figs. 5A-5E). In human PMVEC, ZO-1 mRNA expression was unaffected by stimulation with TNF-α +IL-1β, but it was increased significantly by pretreatment with edaravone or dexamethasone (Fig. 5F). Effects of TNF-α+IL-1β, edaravone, dexamethasone, and L-NMMA on the generation of sICAM-1, sVCAM-1, MCP-1, and IL-8 in human PMVEC. Leukocyte recruitment into inflamed tissues engenders microvascular hyperpermeability. Therefore, the effects of TNF-α+IL-1β, edaravone, dexamethasone, and L-NMMA on the production of sICAM-1, sVCAM-1, MCP-1, and IL-8 in human PMVEC were examined. Treatment of human PMVEC with TNF-α+ IL-1β for longer than 6h caused a significant increase in the production of sICAM-1 and sVCAM-1 in the supernatant compared with the non-stimulated cells. The levels of MCP-1 and IL-8 were increased significantly after 3h and 6h, respectively, by the administration of TNF-α+IL-1β(Fig. 6). We examined the inhibitory effects of pretreatment with edaravone, dexamethasone, and L-NMMA for 24h on the production of adherence molecules and chemokines. The sICAM-1 and sVCAM-1 production tended to be decreased by these pretreatments, but the differences were not significant. Pretreatment with dexamethasone significantly inhibited the production of MCP-1 and IL-8 induced by TNF-α+IL-1β
Various diseases including viral pneumonia, sepsis, and acute pancreatitis can cause ARDS, a state of pulmonary edema caused by the influx of protein-rich edema fluid into the air spaces. ARDS can also occur as a consequence of endothelial and epithelial injury [5]. Microvascular endothelial cells respond to local changes in biological needs caused by inflammation. These cells play an important role in the control of exchange of leukocytes and fluids between the lung microvessels and alveoli [23, 24]. The cytokine profiles of suctioned pulmonary secretions from children infected with influenza A/H1N1pdm revealed that TNF-α, IL-1βand other inflammatory cytokine concentrations were markedly higher on the fifth day after hospitalization than on the first day [25]. It has been reported that influenza A/H1N1pdm viruses replicate efficiently in the lungs of infected mice, ferrets, and non-human primates. These viruses can cause remarkable pathology in the lungs [4]. Consequently, locally released inflammatory cytokines in the lung are increased in influenza A/H1N1pdm pneumonia, and are likely to contribute to lung microvascular hyperpermeability. Our experiments showed that stimulation with inflammatory cytokines (TNF-α+IL-1β, 10ng/ml in each) for 24h significantly increased dextran leakage across human PMVEC. Pretreatment with edaravone, dexamethasone, or L-NMMA significantly inhibited the increase of permeability caused by these cytokines in the endothelial cells to the same extent. Stimulation with inflammatory cytokines caused perturbation of VE-cadherin, a major component of the adherence
Discussion
junction [8]. The finding that pretreatment with edaravone, dexamethasone, or L-NMMA inhibited the cytokine-induced perturbation of VE-cadherin suggests that these medications have the effect of holding VE-cadherin. Differential results were observed in terms of the protein levels and mRNA expression of VE-cadherin: although no remarkable change of the mRNA expression was observed, stimulation with inflammatory cytokines led to a decrease in VE-cadherin protein. Pretreatment with edaravone, dexamethasone, or L-NMMA inhibited the effect. This discrepancy points to indirect effects on VE-cadherin expression rather than to direct transcriptional regulation of the VE-cadherin gene. The results also showed that ZO-1 mRNA and near-circumferential expression were increased by pretreatment with edaravone or dexamethasone. These results support the contention that edaravone and dexamethasone can reinforce a tight junction through the enhancement of ZO-1 expression [26, 27]. Pretreatment with edaravone or dexamethasone might contribute to enhancement of the tight junction, resulting in the inhibition of hyperpermeability in human PMVEC.
Several previous studies have revealed that edaravone increases transmonolayer electrical resistance by enhancing the expression of the adherence junction protein [28]. Edaravone prevents the increase of permeability under oxidative stress [29] in human umbilical vein endothelial cells. Dexamethasone increases VE-cadherin protein levels and rearranges the cytoskeleton [30]. The present study provided similar results that edaravone and dexamethasone prevented hyperpermeability in human PMVEC under cytokine stimulation. Our experiments also showed that stimulation with cytokines increased the mRNA expression and protein levels of ICAM-1, VCAM-1, MCP-1, and IL-8, similar to human dermal microvascular endothelial cells [31, 32]. Pretreatment with edaravone or dexamethasone showed a tendency to inhibit the expression of ICAM-1 and VCAM-1, although the effect was not significant. Pretreatment with dexamethasone reduced MCP-1 and IL-8 significantly. These results present the possibility that edaravone and dexamethasone prevent the increase of vascular permeability partly through the inhibition of leukocyte recruitment into inflamed tissues. Our recent report described that administration of TNF-α enhances the expression of MMP-9, which dissolves type IV collagen in the brain endothelial cells of mice, and results in disorders of the bloodbrain barrier [33]. However, administration of TNF-α+IL-1β induced neither mRNA expression nor secretion of MMP-9 in human PMVEC (data not shown). This discrepancy might have resulted from differences in the species, cell types, experimental conditions, or sensitivity of detection methods. In conclusion, our experiments show that edaravone and dexamethasone can prevent hyperpermeability in human PMVEC stimulated by inflammatory cytokines, possibly through enhancement of the adherence junction, and potentially through other mechanisms as well (Fig. 8). These results might provide
Several previous studies have revealed that edaravone increases transmonolayer electrical resistance by enhancing the expression of the adherence junction protein [28]. Edaravone prevents the increase of permeability under oxidative stress [29] in human umbilical vein endothelial cells. Dexamethasone increases VE-cadherin protein levels and rearranges the cytoskeleton [30]. The present study provided similar results that edaravone and dexamethasone prevented hyperpermeability in human PMVEC under cytokine stimulation. Our experiments also showed that stimulation with cytokines increased the mRNA expression and protein levels of ICAM-1, VCAM-1, MCP-1, and IL-8, similar to human dermal microvascular endothelial cells [31, 32]. Pretreatment with edaravone or dexamethasone showed a tendency to inhibit the expression of ICAM-1 and VCAM-1, although the effect was not significant. Pretreatment with dexamethasone reduced MCP-1 and IL-8 significantly. These results present the possibility that edaravone and dexamethasone prevent the increase of vascular permeability partly through the inhibition of leukocyte recruitment into inflamed tissues. Our recent report described that administration of TNF-α enhances the expression of MMP-9, which dissolves type IV collagen in the brain endothelial cells of mice, and results in disorders of the bloodbrain barrier [33]. However, administration of TNF-α+IL-1β induced neither mRNA expression nor secretion of MMP-9 in human PMVEC (data not shown). This discrepancy might have resulted from differences in the species, cell types, experimental conditions, or sensitivity of detection methods. In conclusion, our experiments show that edaravone and dexamethasone can prevent hyperpermeability in human PMVEC stimulated by inflammatory cytokines, possibly through enhancement of the adherence junction, and potentially through other mechanisms as well (Fig. 8). These results might provide
Several previous studies have revealed that edaravone increases transmonolayer electrical resistance by enhancing the expression of the adherence junction protein [28]. Edaravone prevents the increase of permeability under oxidative stress [29] in human umbilical vein endothelial cells. Dexamethasone increases VE-cadherin protein levels and rearranges the cytoskeleton [30]. The present study provided similar results that edaravone and dexamethasone prevented hyperpermeability in human PMVEC under cytokine stimulation. Our experiments also showed that stimulation with cytokines increased the mRNA expression and protein levels of ICAM-1, VCAM-1, MCP-1, and IL-8, similar to human dermal microvascular endothelial cells [31, 32]. Pretreatment with edaravone or dexamethasone showed a tendency to inhibit the expression of ICAM-1 and VCAM-1, although the effect was not significant. Pretreatment with dexamethasone reduced MCP-1 and IL-8 significantly. These results present the possibility that edaravone and dexamethasone prevent the increase of vascular permeability partly through the inhibition of leukocyte recruitment into inflamed tissues. Our recent report described that administration of TNF-α enhances the expression of MMP-9, which dissolves type IV collagen in the brain endothelial cells of mice, and results in disorders of the bloodbrain barrier [33]. However, administration of TNF-α+IL-1β induced neither mRNA expression nor secretion of MMP-9 in human PMVEC (data not shown). This discrepancy might have resulted from differences in the species, cell types, experimental conditions, or sensitivity of detection methods. In conclusion, our experiments show that edaravone and dexamethasone can prevent hyperpermeability in human PMVEC stimulated by inflammatory cytokines, possibly through enhancement of the adherence junction, and potentially through other mechanisms as well (Fig. 8). These results might provide
Several previous studies
Several previous studies have revealed that edaravone increases transmonolayer electrical resistance byenhancing the expression of the adherence junctionprotein [28]. Edaravone prevents the increase ofpermeability under oxidative stress [29] in humanumbilical vein endothelial cells. Dexamethasoneincreases VE-cadherin protein levels and rearrangesthe cytoskeleton [30]. The present study providedsimilar results that edaravone and dexamethasoneprevented hyperpermeability in human PMVEC undercytokine stimulation.Our experiments also showed that stimulation withcytokines increased the mRNA expression and proteinlevels of ICAM-1, VCAM-1, MCP-1, and IL-8,similar to human dermal microvascular endothelialcells [31, 32]. Pretreatment with edaravone or dexamethasone showed a tendency to inhibit the expression of ICAM-1 and VCAM-1, although the effect wasnot significant. Pretreatment with dexamethasonereduced MCP-1 and IL-8 significantly. These resultspresent the possibility that edaravone and dexamethasone prevent the increase of vascular permeabilitypartly through the inhibition of leukocyte recruitmentinto inflamed tissues.Our recent report described that administration ofTNF-α enhances the expression of MMP-9, whichdissolves type IV collagen in the brain endothelialcells of mice, and results in disorders of the bloodbrain barrier [33]. However, administration ofTNF-α+IL-1β induced neither mRNA expressionnor secretion of MMP-9 in human PMVEC (data notshown). This discrepancy might have resulted fromdifferences in the species, cell types, experimentalconditions, or sensitivity of detection methods.In conclusion, our experiments show that edaravone and dexamethasone can prevent hyperpermeability in human PMVEC stimulated by inflammatorycytokines, possibly through enhancement of the adherence junction, and potentially through other mechanisms as well (Fig. 8). These results might provide
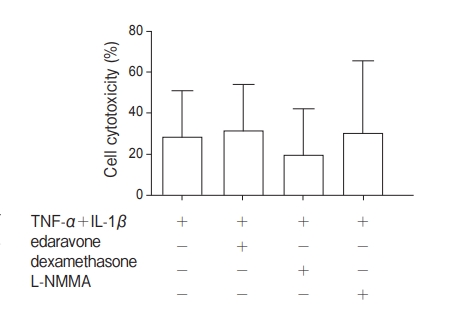
Fig. 3 Effects of edaravone, dexamethasone, and N-monomethyl-L-arginine (L-NMMA) on cell cytotoxicity in human PMVEC stimulated with TNF-α+IL-1β. In evaluation of cell cytotoxicity as determined by the LDH release, no significant differences were found among groups.
expression was unaffected by stimulation with TNF-α +IL-1β, but it was increased significantly by pretreatment with edaravone or dexamethasone (Fig. 5F).
Effects of TNF-α+IL-1β, edaravone, dexamethasone, and L-NMMA on the generation of sICAM-1, sVCAM-1, MCP-1, and IL-8 in human PMVEC.
Treatment of human PMVEC with TNF-α+ IL-1β for longer than 6h caused a significant increase in the production of sICAM-1 and sVCAM-1 in the supernatant compared with the non-stimulated cells. The levels of MCP-1 and IL-8 were increased significantly after 3h and 6h, respectively, by the administration of TNF-α+IL-1β(Fig. 6).
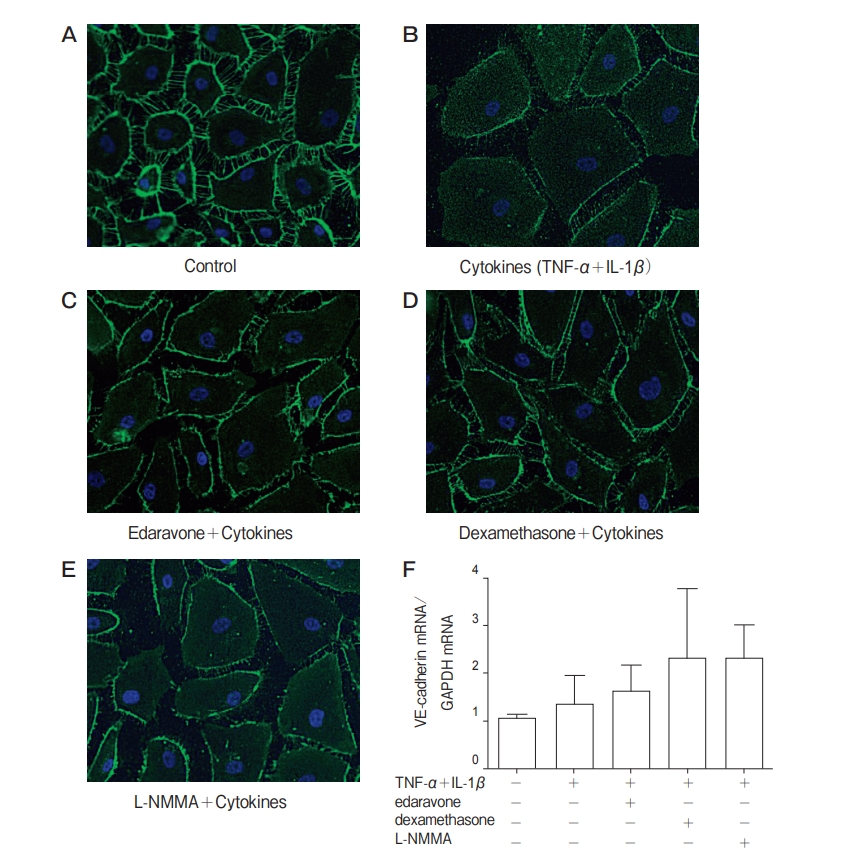
Fig. 4 Effects of TNF-α+IL-1β, edaravone, dexamethasone, and N-monomethyl-L-arginine (L-NMMA) on the expression of vascular endothelial (VE)-cadherin in human PMVEC. For immunofluorescence analysis, human PMVEC were treated with culture medium alone (A), with TNF-α+IL-1β for 24h (B), pretreatment with edaravone (C), with dexamethasone (D), with L-NMMA (E) for 24h and treatment with TNF-α+IL-1β for 24 h. TNF-α+IL-1β reduced staining for VE-cadherin. Pretreatment with edaravone, dexamethasone, or L-NMMA apparently inhibited this effect. VE-cadherin mRNA (F) was not altered significantly by these chemicals. Representative immunofluorescence studies are shown.
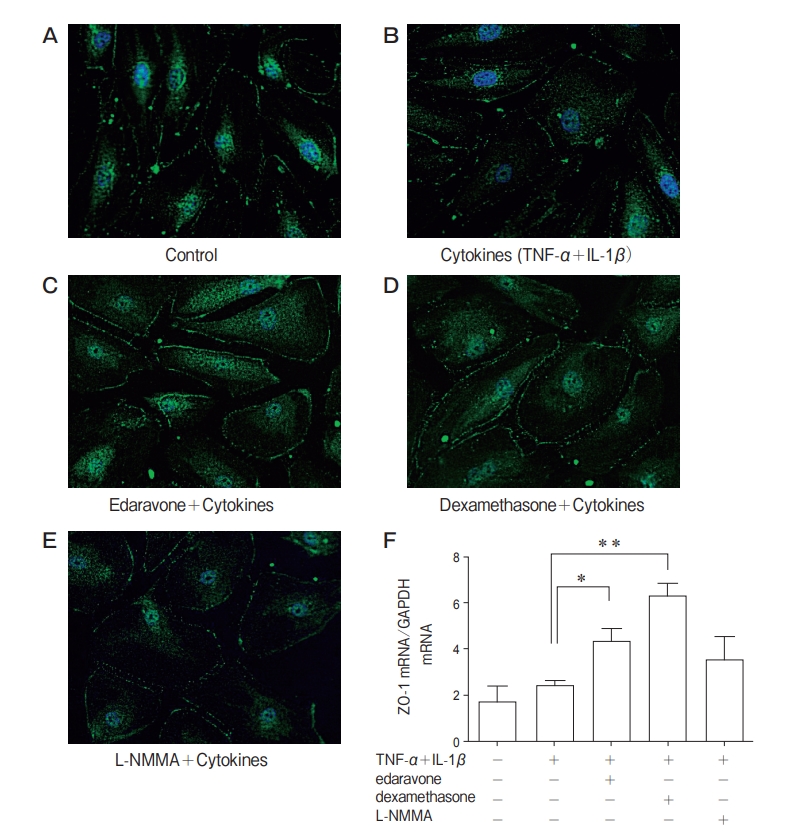
Fig. 5 Effects of TNF-α+IL-1β, edaravone, dexamethasone, and N-monomethyl-L-arginine (L-NMMA) on the expression of zonula occuldens-1 protein (ZO-1) in human PMVEC. For immunofluorescence analysis, human PMVEC were treated with culture medium alone (A), with TNF-α+IL-1β for 24h (B), pretreatment with edaravone (C), with dexamethasone (D), with L-NMMA (E) for 24h and treatment with TNF-α+IL-1β for 24 h. No change of ZO-1 expression was found from TNF-α+IL-1β treatment. Pretreatment with edaravone or dexamethasone increased ZO-1 expression compared with control or cytokine group. Representative immunofluorescence studies are shown. ZO-1 mRNA (F) was enhanced by pretreatment with edaravone or dexamethasone compared with control and TNF-α+IL-1β group. Data are presented as mean ± SD (n=4 in each group). *p<0.05 in comparison with TNF-α+IL-1β group. **p<0.01 in comparison with TNF-α+IL-1βgroup.
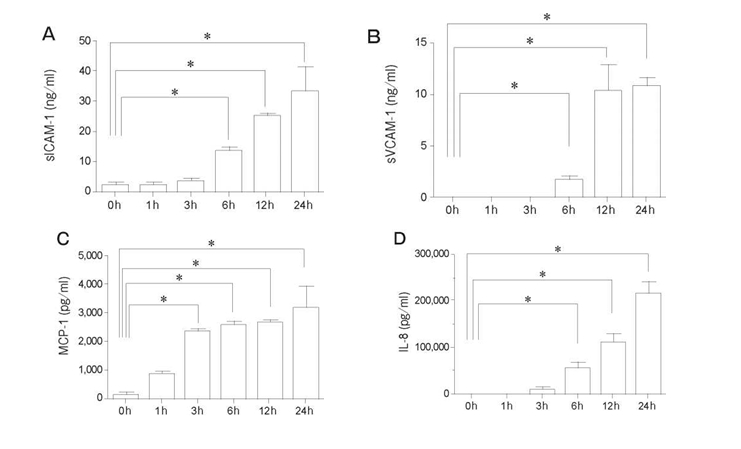
Fig. 6 Effects of TNF-α+IL-1βon the secretion of sICAM-1, sVCAM-1, MCP-1, and IL-8 in human PMVEC. Treatment with TNFα+IL-1β(10ng/ml) for 6h significantly increased the secretion of sICAM-1 (A), sVCAM-1 (B), and IL-8 (D). The secretion of MCP-1 (C) was increased by stimulation for 3h. Data are presented as mean ± SD (n=4 in each group). *p<0.05 in comparison with 0 h in each group. sICAM-1, soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular cell adhesion molecule-1; MCP-1, monocyte chemoattractant protein-1; IL-8, interleukin-8.
Discussion
Various diseases including viral pneumonia, sepsis, and acute pancreatitis can cause ARDS, a state of pulmonary edema caused by the influx of protein-rich edema fluid into the air spaces. ARDS can also occur as a consequence of endothelial and epithelial injury [5]. Microvascular endothelial cells respond to local changes in biological needs caused by inflammation. These cells play an important role in the control of exchange of leukocytes and fluids between the lung microvessels and alveoli [23, 24].
Fig. 5 Effects of TNF-α+IL-1β, edaravone, dexamethasone, and N-monomethyl-L-arginine (L-NMMA) on the expression of zonula occuldens-1 protein (ZO-1) in human PMVEC. For immunofluorescence analysis, human PMVEC were treated with culture medium alone (A), with TNF-α+IL-1β for 24h (B), pretreatment with edaravone (C), with dexamethasone (D), with L-NMMA (E) for 24h and treatment with TNF-α+IL-1β for 24 h. No change of ZO-1 expression was found from TNF-α+IL-1β treatment. Pretreatment with edaravone or dexamethasone increased ZO-1 expression compared with control or cytokine group. Representative immunofluorescence studies are shown. ZO-1 mRNA (F) was enhanced by pretreatment with edaravone or dexamethasone compared with control and TNF-α+IL-1β group. Data are presented as mean ± SD (n=4 in each group). *p<0.05 in comparison with TNF-α+IL-1β group. **p<0.01 in comparison with TNF-α+IL-1βgroup.
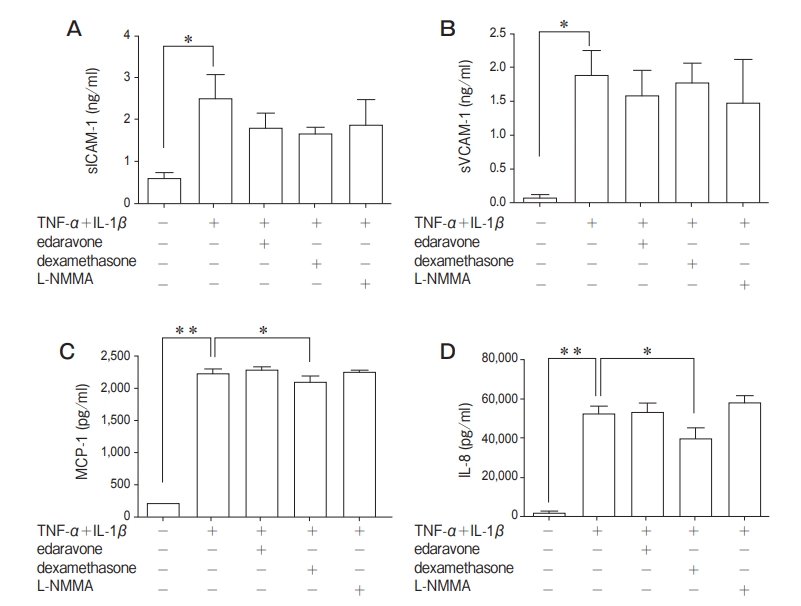
Fig. 7 Effects of edaravone, dexamethasone, and N-monomethyl-L-arginine (L-NMMA) on the secretion of sICAM-1, sVCAM-1, MCP-1, and IL-8 in the human PMVEC stimulated with TNF-α+IL-1β(10ng/ml in both). Up-regulation of sICAM-1 and sVCAM-1 were not suppressed by these chemicals. MCP-1 and IL-8 were suppressed significantly by pretreatment with dexamethasone. Data are presented as mean ± SD (n=4 in each group). *p<0.05, **p<0.01. sICAM-1, soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular cell adhesion molecule-1; MCP-1, monocyte chemoattractant protein-1; IL-8, interleukin-8.
It has been reported that influenza A/H1N1pdm viruses replicate efficiently in the lungs of infected mice, ferrets, and non-human primates. These viruses can cause remarkable pathology in the lungs [4]. Consequently, locally released inflammatory cytokines in the lung are increased in influenza A/H1N1pdm pneumonia, and are likely to contribute to lung microvascular hyperpermeability.
These results might provide 288 Saito et al. Acta Med. Okayama Vol. 69, No. 5 endothelial cell ZO-1 tight junction permeability edaravone, dexamethasone VE-cadherin adherence junction permeability TNF-α IL-1β alveolar cell Fig. 8 Schematic diagram showing TNF-α+IL-1β-induced lung hyperpermeability and the inhibitory effects of edaravone and dexamethasone. cellular- and molecular-level insight into treatment strategies for patients with ARDS and highly pathogenic avian influenza pneumonia, which strongly impair lung function. Edaravone and dexamethasone showed the effect to the same extent. In addition, because edaravone has fewer side effects than dexamethasone [11], edaravone alone or in combination with dexamethasone is expected to be effective for therapy for ARDS in a clinical setting.
Edaravone and dexamethasone showed the effect to the same extent. In addition, because edaravone has fewer side effects than dexamethasone [11], edaravone alone or in combination with dexamethasone is expected to be effective for therapy for ARDS in a clinical setting.
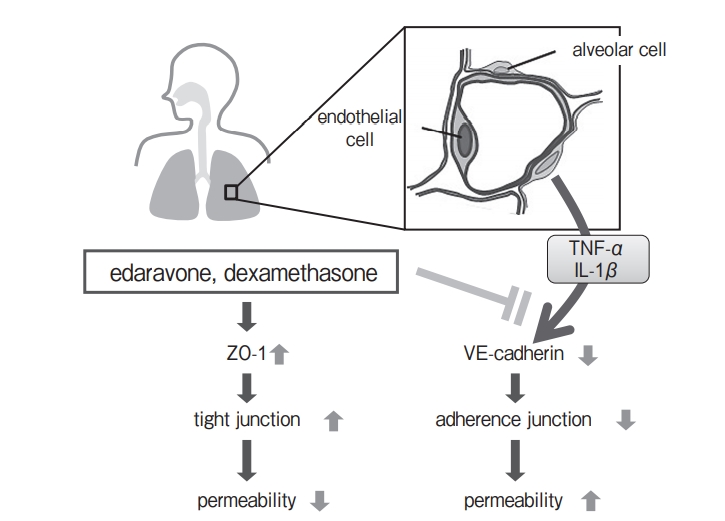
Fig. 8 Schematic diagram showing TNF-α+IL-1β-induced lung hyperpermeability and the inhibitory effects of edaravone and dexamethasone.
References
- Rello J and Pop-Vicas A: Clinical review: primary influenza viral pneumonia. Crit Care (2009) 13 : 235.
- Tran D, Vaudry W, Moore DL, Bettinger JA, Halperin SA, Schiefele DW and Aziz S; IMPACT investigators: Comparison of children hospitalized with seasonal versus pandemic influenza A, 2004-2009. Pediatrics (2012) 130 : 397-406.
- Pabst D, Kuehn J, Schuler-Luettmann S, Wiebe K and Lebiedz P: Acute Respiratory Distress Syndrome as a presenting manifestation in young patients infected with H1N1 influenza virus. Eur J Intern Med (2011) 22 : e119-e124.
- Itoh Y, Shinya K, Kiso M, Watanabe T, Sakoda Y, Hatta M, Muramoto Y, Tamura D, Sakai-Tagawa Y, Noda T, Sakabe S, Imai M, Hatta Y, Watanabe S, Li C, Yamada S, Fujii K, Murakami S, Imai H, Kakugawa S, Ito M, Takano R, Iwatsuki- Horimoto K, Shimojima M, Horimoto T, Goto H, Takahashi K, Makino A, Ishigaki H, Nakayama M, Okamatsu M, Warshauer D, Shult PA, Saito R, Suzuki H, Furuta Y, Yamashita M, Mitamura K, Nakano K, Nakamura M, Brockman-Schneider R, Mitamura H, Yamazaki M, Sugaya N, Suresh M, Ozawa M, Neumann G, Gern J, Kida H, Ogasawara K and Kawaoka Y: In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature (2009) 460 : 1021-1025.
- Ware LB and Matthay MA: The acute respiratory distress syndrome. N Engl J Med (2000) 342 : 1334-1349.
- Fukuhra S, Sakurai A, Yamagishi A, Sako K and Mochizuki N: Vascular endothelial cadherin-mediated cell-cell adhesion regulated by a small GTPase, Rap1. J Biochem Mol Biol (2006) 39 : 132-139.
- Dejana E, Corada M and Lampugnani MG: Endothelial cell-to-cell junctions. FASEB J (1995) 9 : 910-918.
- Dejana E, Orsenigo F and Lampugnani MG: The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci (2008) 121 : 2115-2122.
- Wittchen ES: Endothelial signaling in paracellular and transcellular leukocyte transmigration. Front Biosci (Landmark Ed) (2009) 14 : 2522-2545.
- Ohta Y, Takamatsu K, Fukushima T, Ikegami S, Takeda I, Ota T, Goto K and Abe K: Efficacy of the free radical scavenger, edara- vone, for motor palsy of acute lacunar infarction. Intern Med (2009) 48 : 593-596.
- Kikuchi K, Uchikado H, Miyagi N, Morimoto Y, Ito T, Tancharoen S, Miura N, Miyata K, Sakamoto R, Kikuchi C, Iida N, Shiomi N, Kuramoto T and Kawahara K: Beyond neurological disease: new targets for edaravone (Review). Int J Mol Med (2011) 28 : 899-906.
- Tajima S, Bando M, Ishii Y, Hosono T, Yamasawa H, Ohno S, Takada T, Suzuki E, Gejyo F and Sugiyama Y: Effects of edara- vone, a free-radical scavenger, on bleomycin-induced lung injury in mice. Eur Respir J (2008) 32 : 1337-1343.
- Tajima S, Soda M, Bando M, Enomoto M, Yamasawa H, Ohno S, Takada T, Suzuki E, Gejyo F and Sugiyama Y: Preventive effects of edaravone, a free radical scavenger, on lipopolysaccharide- induced lung injury in mice. Respirology (2008) 13 : 646-653.
- Yang T, Mao YF, Liu SQ, Hou J, Cai ZY, Hu JY, Ni X, Deng XM and Zhu XY: Protective effects of the free radical scavenger edaravone on acute pancreatitis-associated lung injury. Eur J Pharmacol (2010) 630 : 152-157.
- Yang T, Zhang J, Sun L, Zhu X, Li J, Wang J, Chen H, Bao R, Deng X, Hou J and Liu Y: Combined effects of a neutrophil elastase inhibitor (sivelestat sodium) and a free radical scavenger (edaravone) on lipopolysaccharide-induced acute lung injury in rats. Inflamm Res (2012) 61 : 563-569.
- Sibbald WJ, Anderson RR, Reid B, Holliday RL and Driedger AA: Alveolo-capillary permeability in human septic ARDS. Effect of high-dose corticosteroid therapy. Chest (1981) 79 : 133-142.
- Matsuo N: The role of intrapulmonary nitric oxide generation in the development of adult respiratory distress syndrome. Surg Today (1999) 29 : 1068-1074.
- Maruo N, Morita I, Shirao M and Murota S: IL-6 increases endothelial permeability in vitro. Endocrinology (1992) 131 : 710714.
- Zhu YT, Hayashida Y, Kheirkhah A, He H, Chen SY and Tseng SC: Characterization and comparison of intercellular adherent junctions expressed by human corneal endothelial cells in vivo and in vitro. Invest Ophthalmol Vis Sci (2008) 49 : 3879-3886.
- Koch AE, Halloran MM, Haskell CJ, Shah MR and Polverini PJ: Angiogenesis mediated by soluble forms of E-selectin and vascular cell adhesion molecule-1. Nature (1995) 376 : 517-519.
- Haubner F, Lehle K, Munzel D, Schmid C, Birnbaum DE and Preuner JG: Hyperglycemia increases the levels of vascular cellular adhesion molecule-1 and monocyte-chemoattractant-protein-1 in the diabetic endothelial cell. Biochem Biophys Res Commun (2007) 360 : 560-565.
- Lehle K, Haubner F, Munzel D, Birnbaum DE and Preuner JG: Development of a disease-specific model to evaluate endothelial dysfunction in patients with diabetes mellitus. Biochem Biophys Res Commun (2007) 357 : 308-313.
- Jiang MZ, Tsukahara H, Hayakawa K, Todoroki Y, Tamura S, Ohshima Y, Hiraoka M and Mayumi M: Effects of antioxidants and NO on TNF-alpha-induced adhesion molecule expression in human pulmonary microvascular endothelial cells. Respir Med (2005) 99 : 580-591.
- Bogatcheva NV, Zemskova MA, Kovalenkov Y, Poirier C and Verin AD: Molecular mechanisms mediating protective effect of cAMP on lipopolysaccharide (LPS)-induced human lung microvas- cular endothelial cells (HLMVEC) hyperpermeability. J Cell Physiol (2009) 221 : 750-759.
- Kawashima H, Go S, Kashiwagi Y, Morishima Y, Miura T, Ushio M, Nishimata S and Takekuma K: Cytokine profiles of suction pulmonary secretions from children infected with pandemic influenza A (H1N1) 2009. Crit Care (2010) 14 : 411.
- Onodera H, Arito M, Sato T, Ito H, Hashimoto T, Tanaka Y, Kurokawa MS, Okamoto K, Suematsu N and Kato T: Novel effects of edaravone on human brain microvascular endothelial cells revealed by a proteomic approach. Brain Res (2013) 1534 : 87-94.
- Lutgendorf MA, Ippolito DL, Mesngon MT, Tinnemore D, Dehart MJ, Dolinsky BM and Napolitano PG: Effect of dexamethasone administered with magnesium sulfate on inflammation-mediated degradation of the blood-brain barrier using an in vitro model. Reprod Sci (2014) 21 : 483-491.
- Omori K, Shikata Y, Sarai K, Watanabe N, Wada J, Goda N, Kataoka N, Shikata K and Makino H: Edaravone mimics sphin- gosine-1-phosphate-induced endothelial barrier enhancement in human microvascular endothelial cells. Am J Physiol Cell Physiol (2007) 293 : C1523-C1531.
- Morozumi J, Mishima S, Ohta S, Fujikawa T, Sasaki H, Noda M and Yukioka T: The role of edaravone on the impairment of endothelial barrier function induced by acute oxidative stress in cultured human umbilical vein endothelial cell monolayer. J Trauma (2005) 59 : 570-574; discussion 574.
- Blecharz KG, Drenckhahn D and Forster CY: Glucocorticoids increase VE-cadherin expression and cause cytoskeletal rearrangements in murine brain endothelial cEND cells. J Cereb Blood Flow Metab (2008) 28 : 1139-1149.
- Jiang MZ, Tsukahara H, Ohshima Y, Sato S, Todoroki Y, Hiraoka M and Mayumi M: Effects of antioxidant and nitric oxide on chemokine production in TNF-alpha-stimulated human dermal microvascular endothelial cells. Free Radic Res (2004) 38 : 473480.
- Jiang MZ, Tsukahara H, Ohshima Y, Todoroki Y, Hiraoka M, Maeda M and Mayumi M: Effects of antioxidants and nitric oxide on TNF-alpha-induced adhesion molecule expression and NF-kappaB activation in human dermal microvascular endothelial cells. Life Sci (2004) 75 : 1159-1170.
- Tsuge M, Yasui K, Ichiyawa T, Saito Y, Nagaoka Y, Yashiro M, Yamashita N and Morishima T: Increase of tumor necrosis factoralpha in the blood induces early activation of matrix metalloprotei- nase-9 in the brain. Microbiol Immunol (2010) 54 : 417-424.
Departments of Pediatrics and Virology, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama 700-8558, Japan