Acute Kidney Injury and Edaravone in Acute Ischemic Stroke: The Fukuoka Stroke Registry
Background: A free radical scavenger, edaravone, which has been used for the treatment of ischemic stroke, was reported to cause acute kidney injury (AKI) as a fatal adverse event. The aim of the present study was to clarify whether edaravone is associated with AKI in patients with acute ischemic stroke. Methods: From the Fukuoka Stroke Registry database, 5689 consecutive […]
Posted: International Neurological Journal, №3 (105), 2019
The search for new methods and drugs to struggle with cerebral stroke and its complications
The scientific-practical conference “Opportunities and Achievements of Modern Pharmacotherapy in the Practice of a Neurologist” held in Kharkov on March 14-15, 2019. It was organized by Association of Neurologists, Psychiatrists and Narcologists of Ukraine, Institute of Neurology, Psychiatry and Narcology of the National Academy of Medical Sciences of Ukraine, Kharkiv National University named after V.N. […]
Posted: Health of Ukraine. Thematic issue "Neurology, Psychiatry, Psychotherapy" No. 2, 2019.
How is edaravone effective against acute ischemic stroke and amyotrophic lateral sclerosis?
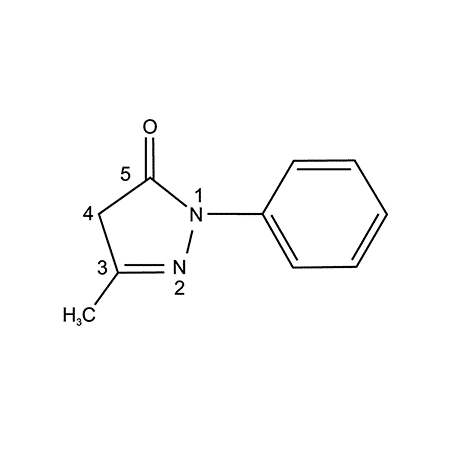
Abstract. Edaravone isa low-molecular-weight antioxidant drug targeting peroxyl radicals among many types of reactive oxygen species. Because of its amphiphilicity, it scavenges both lipid- and water-soluble peroxyl radicals by donating an electron to the radical. Thus, it inhibits the oxidation of lipids by scavenging chain-initiating water-soluble peroxyl radicals and chain-carrying lipid peroxyl radicals. In 2001, it […]
Posted: J. Clin. Biochem. Nutr. January 2018. Vol. 62, № 1. Р. 20-38
Ukrainian legislation on the prevention and treatment of rare (orphan) diseases
From 01 January 2015, amendments to the Fundamentals of Legislation of Ukraine on Health Care implemented by the Law of Ukraine No. 1213-VII dated 15 April 2014, have become effective. According to the implemented amendments to the legislation, patients with rare (orphan) diseases shall be provided, on a continuous and free basis, with medicinal products for the […]
Posted: Відомості Верховної Ради (ВВР), 2014, № 26; Постанова Кабінету міністрів України №160 від 31.03.2015 р.
Extended revised ALS Scale (ALSFRS-R)
Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS) is a tool for monitoring the progression of disability in patients with amyotrophic lateral sclerosis (ALS). The disadvantage of ALSFRS is that its rating of the bulbar functions and limb functionality is overweighted compared to the rating of respiratory dysfunction. A revised ALS functional rating scale (ALSFRS-R) retains the properties […]
Posted: version of May 2015.
Safety and efficacy of edarowon in patients with amyotrophic lateral sclerosis: a randomized, double-blind, placebo-controlled study
This randomized double-blind parallel trial (Phase III) was attended by patients aged 20-75 years with grade 1 or 2 ALS from 31 hospitals in Japan. Patients had a score of at least 2 for each of all 12 points of ALSFRS-R, a forced vital lung capacity of ≥ 80% and an established or suspected ALS […]
Posted: сайт thelancet.com/neurology, 15 травня 2017.
In 2017, the FDA approved a new drug for the treatment of lateral amyotrophic sclerosis (press release, in English)
On 05 May 2017, US Food and Drug Administration (FDA) approved edaravone as an officially recommended drug product for the treatment of patients with amyotrophic lateral sclerosis (ALS). The U.S. Food and Drug Administration today approved Radicava (edaravone) to treat patients with amyotrophic lateral sclerosis (ALS), commonly referred to as Lou Gehrig’s disease. “After learning […]